Better affordability of high-quality medicines
We drive early market entries at best prices
Get a reliable, flexible, and cost-efficient supply!
We, at TIEFEENBACHER PHARMACEUTICALS, strongly believe that our high-quality medicines should be affordable for all patients irrespective of their income level. That´s why we strive for these two goals:
- Achieving an earliest possible market entry for our customers
AET is well known for its highly rated research & development competence, quality excellence, and regulatory expertise – being the foundation for a constant track record of first-to-file dossiers. A professional project management and an outstanding intellectual property guidance are further important pillars to ensure that our first-to-market strategies are successful. - Driving efficiency in our supply chains to offer best possible prices
We drive efficiency by keeping our supply chains simple and reaching economies of scale in our own manufacturing sites. This results in best value for money for our customers. Modern supply chain solutions and a lean management approach ensure that our affordable products reach the right place in the right condition and quantity on time. All our pharmaceutical production plants are equipped with up-to-date technologies, highly qualified professionals, and the highest safety standards compliant with Good Manufacturing Practice (GMP). Apart from the most important governmental approvals, our sites are periodically inspected by leading pharmaceutical companies.

How we drive better affordability of high-quality medicines
Our success factors for early market entries at best prices
Driving efficient supply chains and early market entries in multiple disease areas for affordable healthcare worldwide
highly rated regulatory expertise
R&D and quality assurance excellence
constant generation of first-to-file-dossiers
efficiency-driven supply chain management
highly rated regulatory expertise
R&D and quality assurance excellence
constant generation of first-to-file-dossiers
efficiency driven supply chain management
economies of scale in own manufacturing sites
reliable and flexible supply
professional project management
outstanding intellectual property guidance
economies of scale in own manufacturing sites
reliable and flexible supply
professional project management
outstanding intellectual property guidance
Our Products: High quality generic, value added, and innovative medicines for global markets
TIEFENBACHER PHARMACEUTICALS develops and commercializes generic, value-added and innovative pharmaceutical products
Driven by our purpose of bringing high-quality medicines within affordable reach to patients around the world, we provide over 180+ products across various therapeutic categories in 10+ dosage forms – and additional medical devices. We are not limited to specific disease areas – whether for widespread or for orphan diseases. We are developing in-house products that address specific therapeutic needs in unique formulations of prescription drugs (Rx) as well as over-the-counter (OTC) medicines – for the benefit of patients worldwide. Furthermore, we are committed for growth and innovation by offering a well-defined and specialized portfolio of highly potent drugs.
Diseases Areas
Oncology
Respiratory
Neuroscience
Cardiovascular, Renal and Metabolism
Immunology, Hepatology and Dermatology
Infectious Diseases
Ophthalmology
Dosages Forms
Tablets
Capsule
Powder
Drops
Inhaler
Transdermal Patches
Eye drops
Additional
Medical Devices
Our Products: High quality innovative and generic medicins for global market
TIEFENBACHER PHARMACEUTICALS is developing and commercializing value-added medicine and generic pharmaceutical products
Driven by our purpose of bringing high-quality medicines within affordable reach to patients through value-added medicine and generic pharmaceutical products around the world, we provide over 180+ products across various therapeutic categories in 40+ dosage forms – and additional medical devices and drug device combinations. To make healthcare better affordable globally, we are deepening our presence in the key markets of Europe, US, and Asia, and in other emerging markets.
TIEFENBACHER PHARMACEUTICALS focuses on the development, manufacturing and commercialization of high-quality pharmaceutical products. We are proud to be one of the few pharmaceutical companies in Germany that offer a global marketplace. Our in-house R&D organization is focusing on the development and manufacturing of drug products and is the basis of our vertically integrated business approach.
Diseases Areas
Oncology
Respiratory
Neuroscience
Cardiovascular, Renal and Metabolism
Immunology, Hepatology and Dermatology
Infectious Diseases
Ophthalmology
Dosages Forms
Tablets
Capsule
Powder
Drops
Inhaler
Transdermal Patches
Eye drops
Additional
Medical Devices
For further information on medical devices
and drug device combination, please have a look here.
Our Products: High quality innovative and generic medicins for global market
TIEFENBACHER PHARMACEUTICALS is developing and commercializing value-added medicine and generic pharmaceutical products
Driven by our purpose of bringing high-quality medicines within affordable reach to patients through value-added medicine and generic pharmaceutical products around the world, we provide over 180+ products across various therapeutic categories in 40+ dosage forms – and additional medical devices and drug device combinations. To make healthcare better affordable globally, we are deepening our presence in the key markets of Europe, US, and Asia, and in other emerging markets.
TIEFENBACHER PHARMACEUTICALS focuses on the development, manufacturing and commercialization of high-quality pharmaceutical products. We are proud to be one of the few pharmaceutical companies in Germany that offer a global marketplace. Our in-house R&D organization is focusing on the development and manufacturing of drug products and is the basis of our vertically integrated business approach.
Diseases Areas
Oncology
Respiratory
Neuroscience
Cardiovascular, Renal and Metabolism
Immunology, Hepatology and Dermatology
Infectious Diseases
Ophthalmology
Dosages Forms
Tablets
Capsule
Powder
Drops
Inhaler
Transdermal Patches
Eye drops
Additional
Medical Devices
Driving efficiency in our two own manufacturing sites
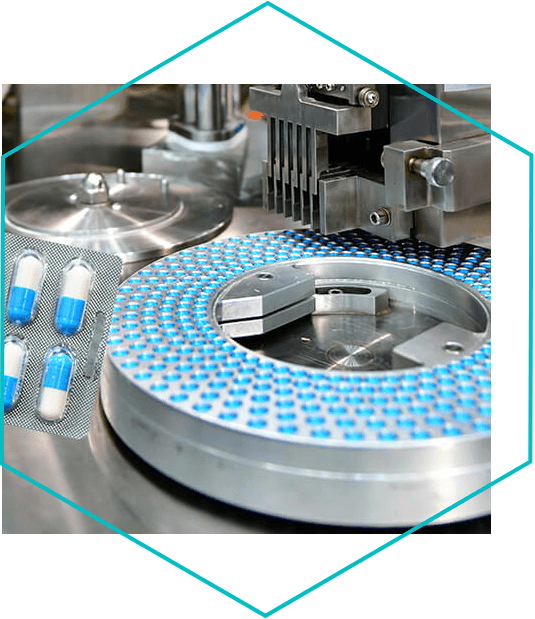
Delorbis Pharmaceuticals – a part of the Tiefenbacher Group
Small batch as well as high-volume productions made in Europe
DELORBIS manufacturing facilities on Cyprus have been designed to accommodate the bulk production and primary and secondary packaging operations of non-sterile oral solid dosage forms (tablets, capsules, and powder suspensions for reconstitution). The production and packaging lines are equipped with sophisticated technology from the first manufacturing step throughout to the last packaging step and are flexible to manage small batch as well as high-volume productions. Thanks to our highly competent personnel as well as the state-of-the-art equipment available in the quality control laboratory, we can also offer to our partners:
- EU batch testing and release of pharmaceutical products
- ICH stability studies at long-term, intermediate, and accelerated conditions for all zones (incl. zone IVb)
- many analytical laboratory services (e. g. chemical testing of raw materials and drug products, method validation/transfer, comparative dissolution profiles) and support from our experienced analysts
- technology transfer
- import/storage/warehousing
Find out more on: http://www.delorbispharma.eu/
AET Laboratories manufacturing site
Our advanced, FDA approved manufacturing site in India
With TIEFENBACHER LABORATORIES, we own a second state-of-the-art manufacturing site, located in Hyderabad, India. Featuring a GMP compliant environment on a 32.000 m² sized plant, TIEFENBACHER LABORATORIES is equipped with the most modern technologies for manufacturing, testing, packing, warehousing, and releasing solid oral dosage forms. With our new high potent manufacturing block we are commited to drive for growth and innovation and are now able to produce a well-defined and specialized portfolio of highly potent drugs.
Quality control is an integral part of TIEFENBACHER LABORATORIES´ manufacturing process. The facility holds the ISO 14001:2014 and ISO 18001:2015 certifications and was successfully inspected by the German BGV, the US FDA authorities, the Russian MIT, and the Turkish Ministry of Health.

We are best practice
Successful day-one-launch of Posaconazole
In 2019 AET successfully launched the first generic product of Posaconazole, a therapeutically equivalent version of the originator product Noxafil®. Studies have demonstrated the satisfactory quality of the product and its bioequivalence to the reference product. Posaconazole is effectively used for the treatment of several fungal infections. With this first-to-market entry in nine European countries, AET achieved nameable savings for the international health care systems and enabled millions of patients a better affordable access to this life-improving treatment. Following our global market entry approach, a launch in about 60 further international countries is already in progress.
Posaconazole has been developed and manufactured in TIEFENBACHER´s own laboratory and manufacturing sites under strictest safety and quality guidelines. An efficiency-driven supply chain approach as well as our highly rated development and regulatory expertise were important pillars for this successful day-one-launch.
Tiefenbacher Pharmaceuticals: Driven by our purpose of bringing high-quality medicines to patients worldwide
We have a product portfolio of more than 180+ products spanning across various therapeutic areas /categories in 10+ dosage froms with unique formulations. Do not hesitate to get in touch for more information about our products and generic development pipeline. Our experts are always available to help.Please contact us by filling out the contact form below and clicking “Send message”. TIEFENBACHER PHARMACEUTICALS will follow up with you personally as soon as possible.


